The Sputnik V vaccine, which was registered in Russia for the first time ever against COVID-19, is now in Turkey!
A Short Brief About Sputnik V
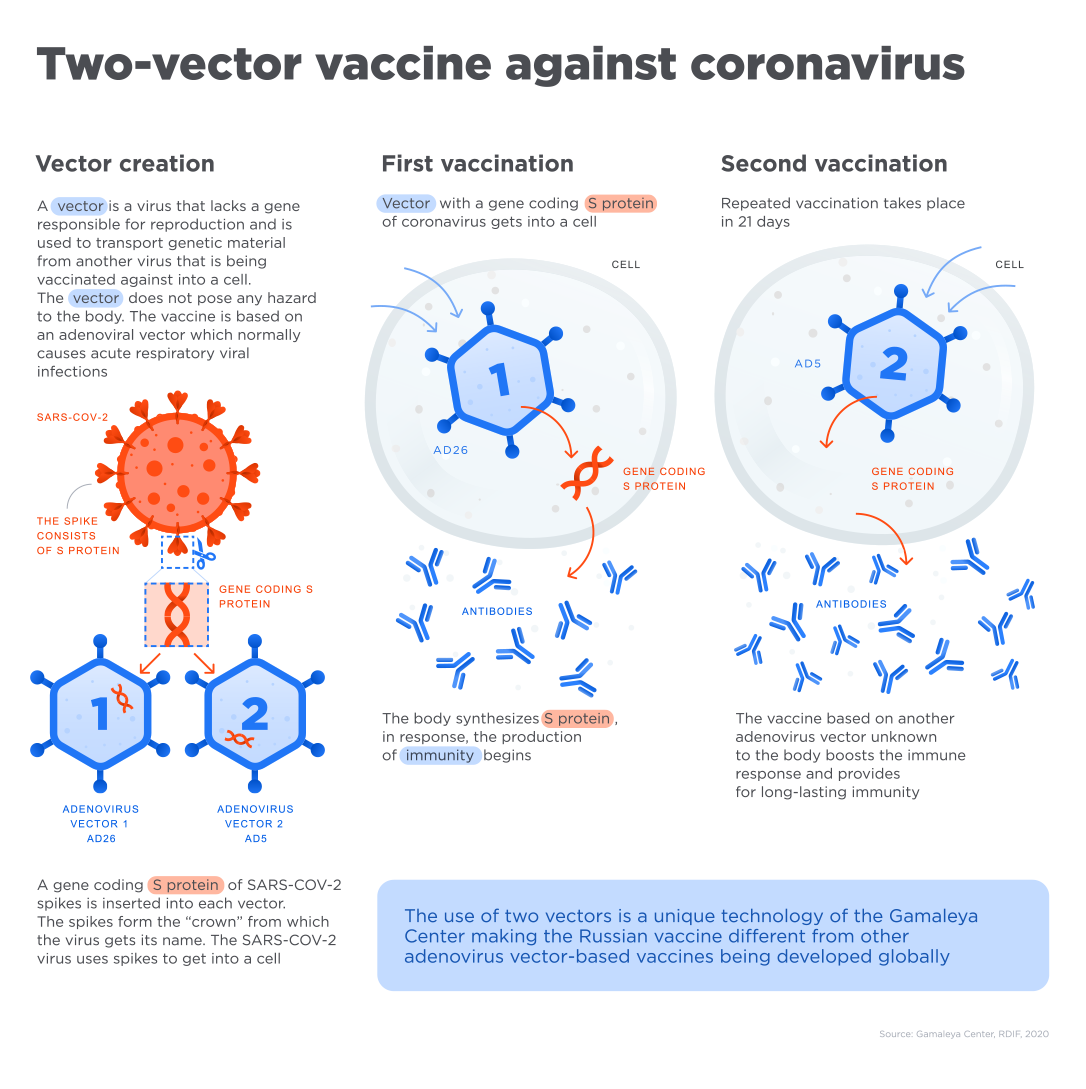
- The Sputnik V vaccine contains adenovirus vectors that cannot replicate in the body. In this respect, it is safe.
Adenovirus-based drugs have been used around the world for more than 50 years. - Human adenovirus vectors of two different serotypes are used for both doses of the vaccine (rAd26 and rAd5).
The advantage of using a different vector in the second dose is to prevent or reduce the effect of the second dose vaccine with the immunity gained against the rAd26 vector that develops after the first vaccination. Thus, the immunity gained is stronger and longer lasting. - Vaccine enables a strong response in antigen-specific cells.
- Its rate of eliciting the IgG (antibody) response in vaccinated participants was 100%.
- Efficacy of Sputnik V is 97.6% based on the analysis of data on the coronavirus infection rate among those in Russia vaccinated with both components of Sputnik V from December 5, 2020 to March 31, 2021.
A long-term immune response is established with prime (first) -boost (second) vaccination.
Adenoviral vectors are a well-studied and safe platform that has been used for vaccination since 1953.
Studies have been conducted with the Sputnik V vaccine on more than 44,000 people worldwide.
Sputnik V vaccine was approved for emergency use in 70 countries. See Countries
Gamaleya National Center for Epidemiology and Microbiology

Founded in 1891, the Gamaleya National Center for Epidemiology and Microbiology, is among the world's leading research institutions.
The Gamaleya Research Center, which has also received an international patent for the Ebola vaccine and recently used the adenovirus vector, has one of the most unique virus collections in the world, as well as its own vaccine production facility.
Two vector-based vaccines against Ebola were successfully developed and registered by the Gamaleya Center in 2015 using the adenovirus vector platform. Another Ebola vaccine was registered in 2020. The vaccines have been officially approved for use by the Russian Ministry of Health. As part of the Phase 3 clinical trial, approximately 2,000 people in Guinea were vaccinated with Ebola in 2017-18. The Gamaleya Research Center has been granted an international patent for the Ebola vaccine.
At the Gamaleya Research Center, adenoviral vectors were used to develop vaccines against influenza and Middle East Respiratory Syndrome (MERS). Clinical trials for both vaccines are currently at an advanced stage.
Clinical Studies
